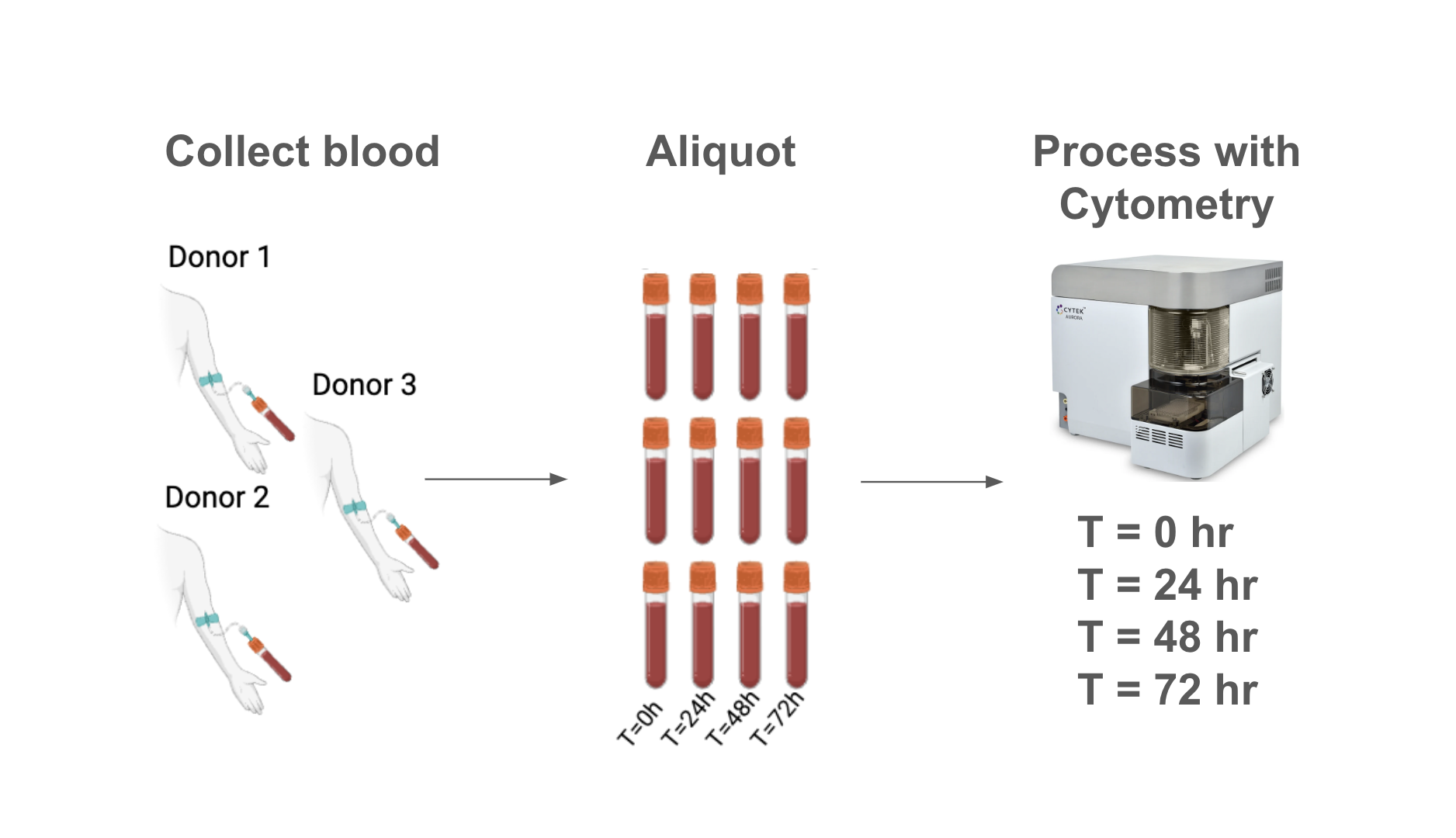
Sample Collection
Whole blood from three healthy donors was collected in sodium heparin vacutainers. Each specimen was split into eight aliquots: four for live processing and staining, and four for fixation.
Timeline
We analyzed samples at time 0 (immediately after draw), 24, 48 and 72 hours after draw.
Live Samples
Samples designated for live processing were diluted in ACK lysis buffer to lyse red blood cells (RBC). Samples were subsequently counted, and stained with a 25-marker antibody cocktail (spectral flow cytometry). After staining, samples were fixed with 1.5% PFA and analyzed.
Analysis Equipment
Analysis was performed on a 5 laser Cytek® Aurora spectral flow cytometer.
Panel Design
Spectral flow cytometry panel design was adapted from Cytek® 25-color Immunoprofiling Assay. The final panel consists of 25 markers with unique spectral signatures and does not contain a viability stain. The panel included markers for:
.png)
.gif)


