TokuTBNK sets the new standard for stability and precision in PK/PD cytometry. Validation data show an average intra-run coefficient of variation (CV) of 6% and average inter-run CV of 9% across 22 immune cell populations. Using Cyto-Chex® BCT tubes for whole blood collection, samples remain stable for up to 7 days at ambient temperature, extending well beyond the 24–48-hour limit common in other CRO workflows.
Performed in Teiko’s CLIA-compliant laboratory, TokuTBNK enables standardized, scalable immune monitoring across global trial sites. The assay delivers consistent, submission-quality PK/PD data that support dose escalation, safety monitoring, and mechanism-of-action studies.
Cytometry provides a critical readout of how therapies affect the immune system, offering insight into drug mechanism, safety, and response. Conventional cytometry workflows, however, are hampered by short processing windows and inconsistent results.
TokuTBNK solves these issues by enabling reproducible cytometry results from stabilized whole blood samples—supporting large, geographically distributed trials.
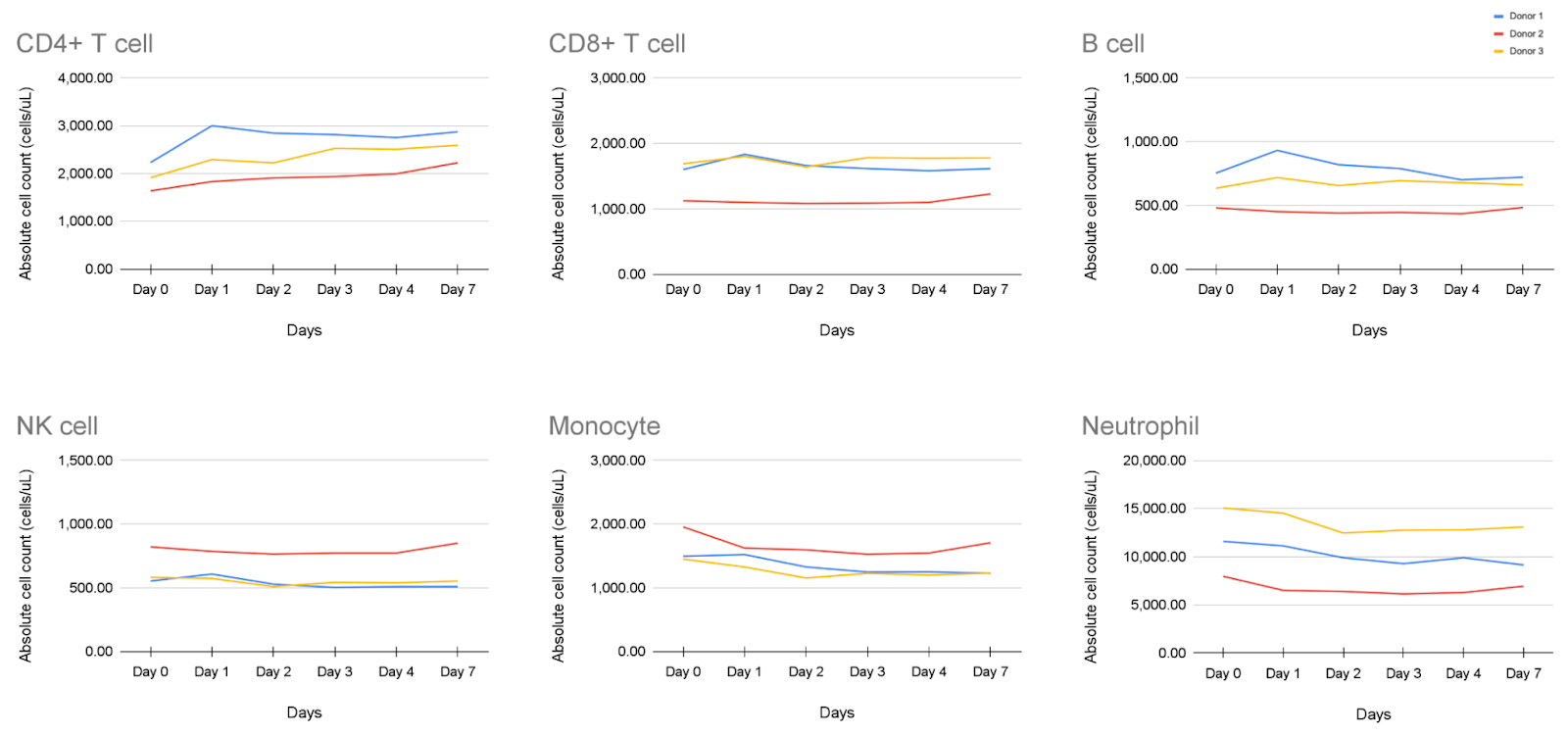
Whole blood collected in Cyto-Chex® BCT tubes from 3 healthy donors was analyzed on Days 0–7. Absolute counts for CD4⁺ T cells, CD8⁺ T cells, B cells, NK cells, monocytes, and neutrophils varied minimally across donors, demonstrating consistent stability and recovery over time.
.png)
Across 22 immune cell populations, average coefficients of variation were 6% (intra-run) and 9% (inter-run). All 22 populations fell below 30% CV within runs, and 95% (21 of 22) fell below 30% CV between runs. Dashed red lines indicate the 30% CV benchmark for cytometry reproducibility.
Blood is collected into Cyto-Chex® BCT tubes at clinical sites, shipped at room temperature, and processed at Teiko’s CLIA-compliant laboratory.
Each sample is stained with a 15-color pan-leukocyte panel to quantify 20+ immune cell populations—including T, B, and NK cells, monocytes, and neutrophils.
Data are analyzed using standardized templates and delivered through Teiko’s Cytometry Dashboard with visualization and AI-assisted analytics.
At Teiko, our mission is simple: to make cytometry simple—reproducible, reliable, and consistent.
We’re addressing every challenge in clinical trial cytometry—from sample collection to analysis—so translational and clinical teams can generate high-quality immune data at scale.