.svg)
Sample Collection + Storage Conditions
Whole blood was collected from four healthy donors and preserved with three common blood preservation methods: Cyto-Chex, Transfix and TokuKit.
TokuKit samples were stored at 4 °C or –80 °C for 7 or 14 days, while Cyto-Chex and Transfix samples were stored at room temperature or 4 °C for 1, 2, 3, or 7 days.
Timeline
After storage, samples were analyzed at multiple timepoints depending on the method:
- Cyto-Chex: 1, 2, 3, and 7 days
- Transfix: 1, 2, 3, and 7 days
- TokuKit: 7 and 14 days
Red Blood Cell Lysis
Samples preserved with Cyto-Chex and Transfix were processed using either ACK or BD lysis prior to staining. Since TokuKit contains its own lysis buffer, no additional ACK or BD lysis step was required.
Panel Design
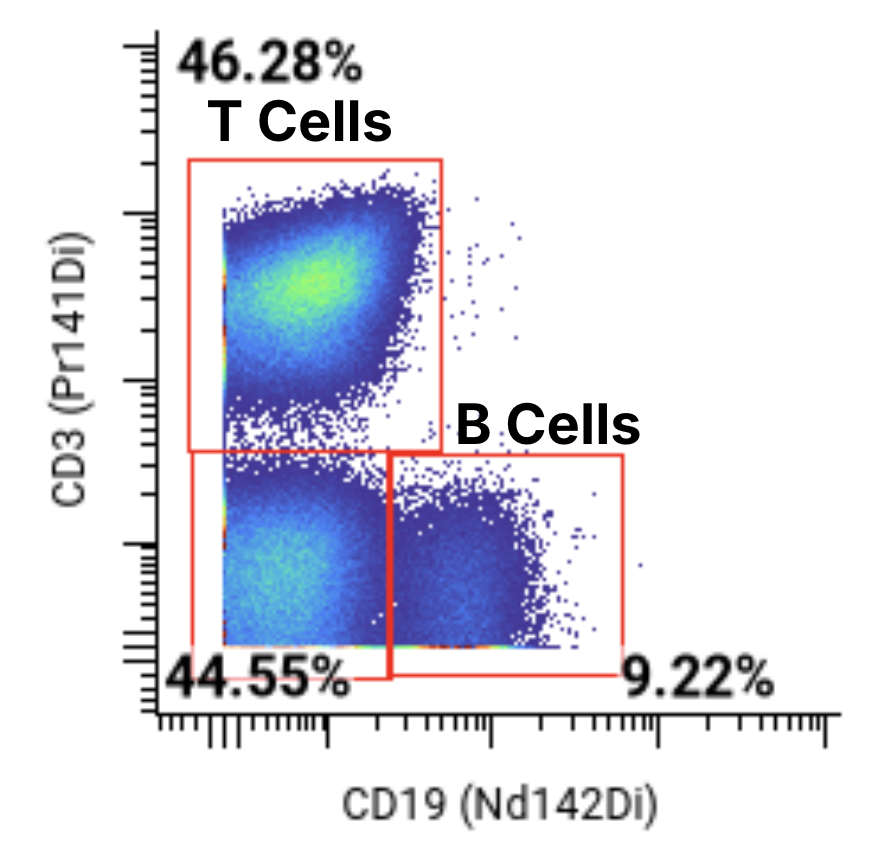
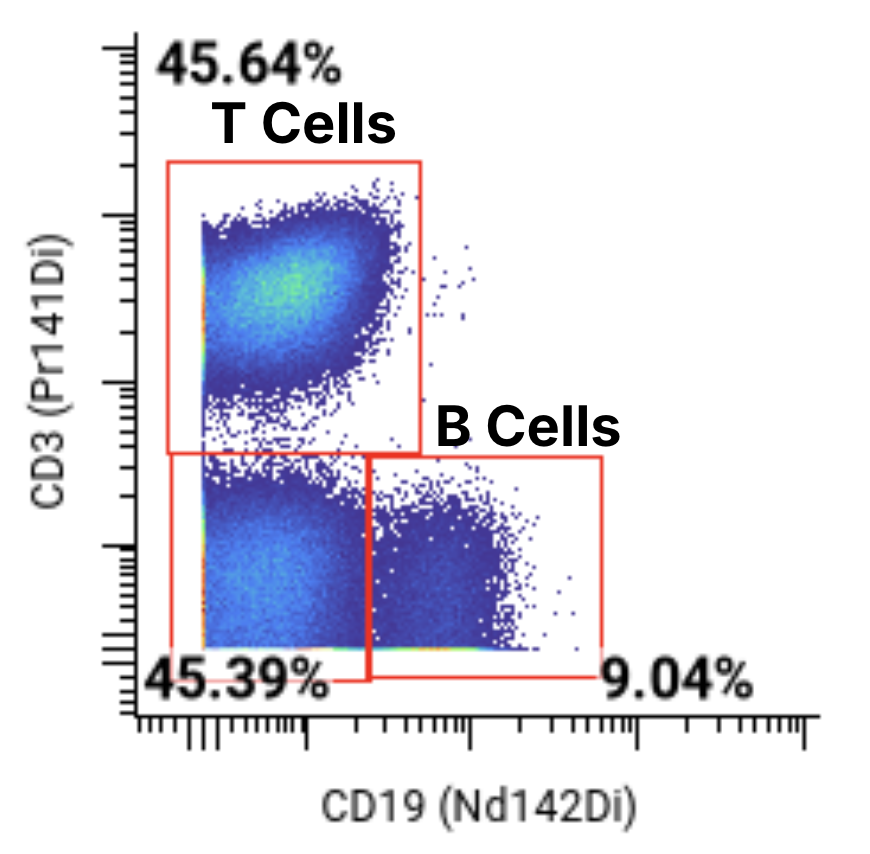
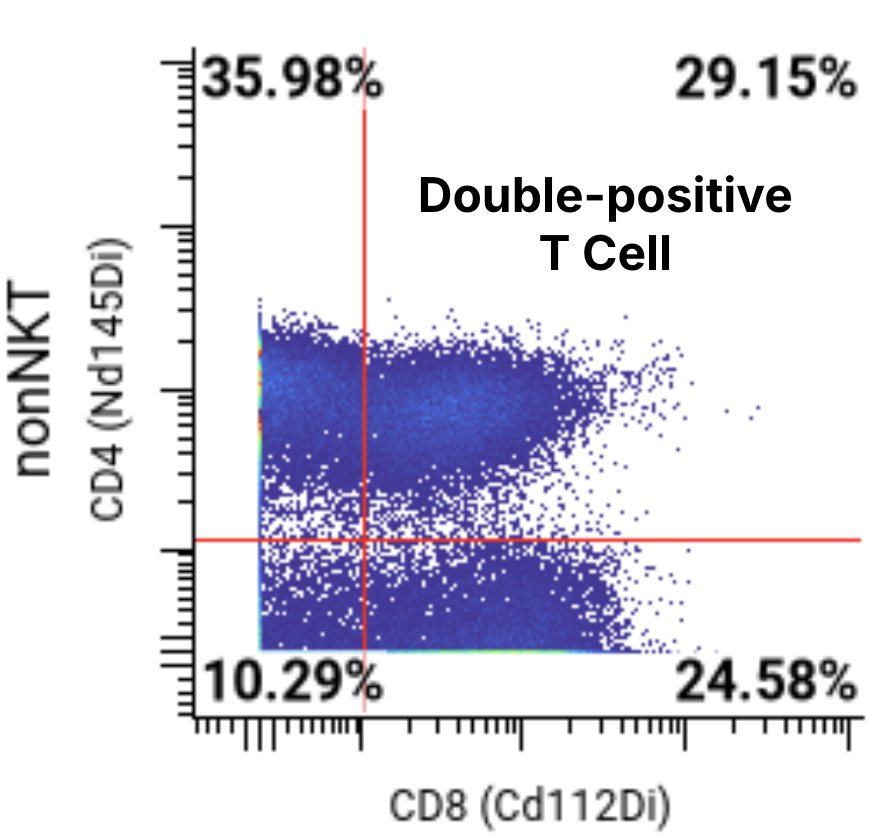
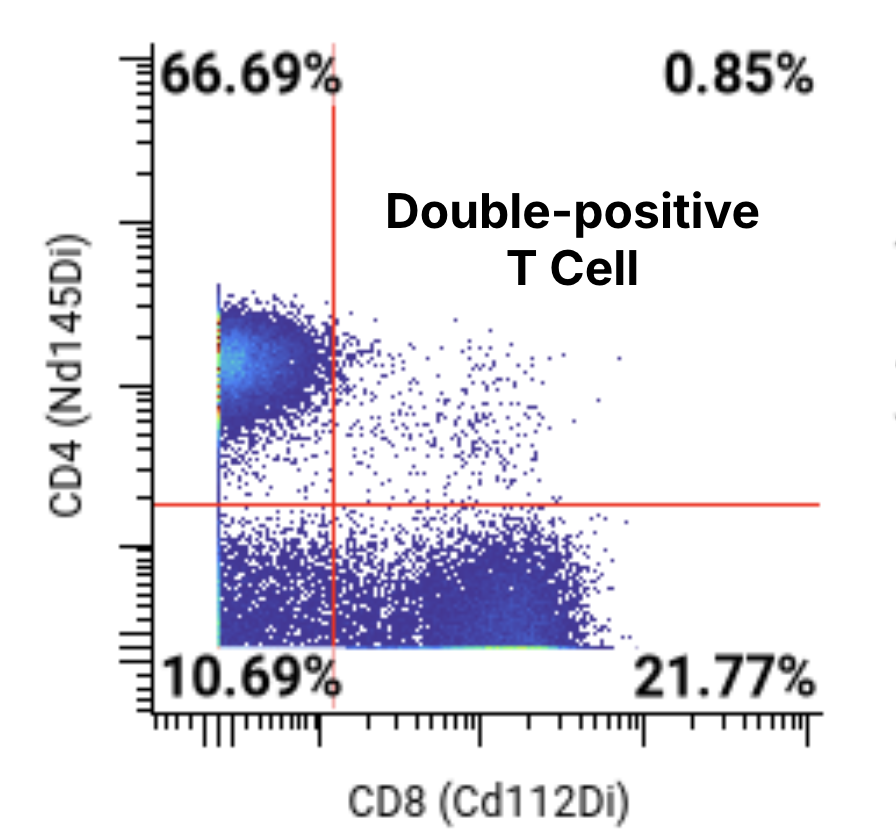
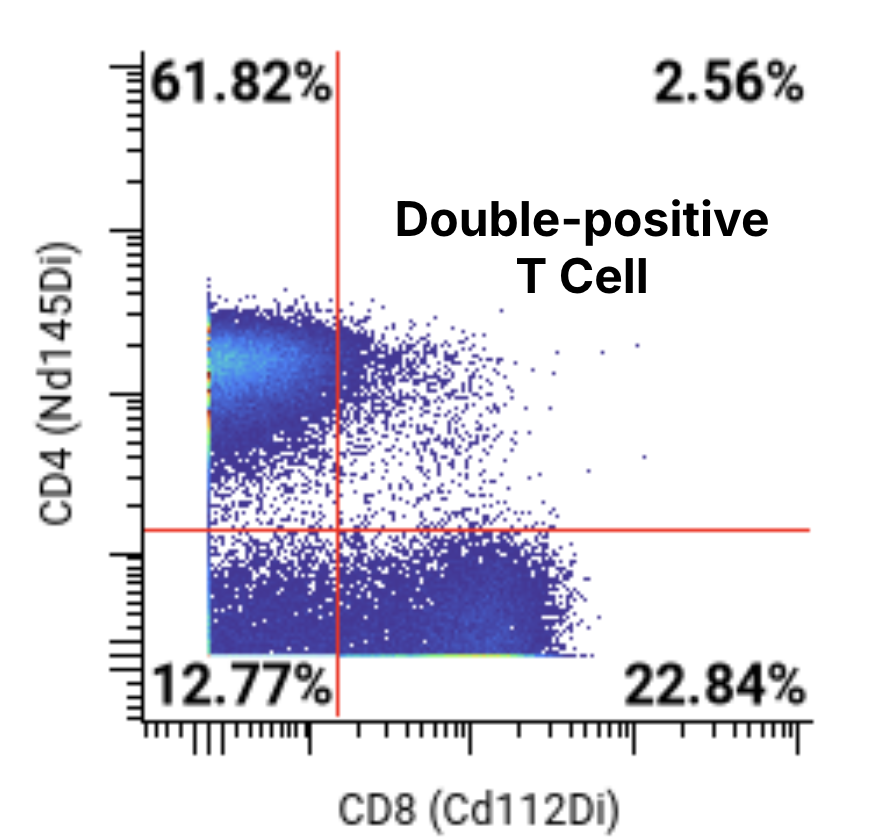
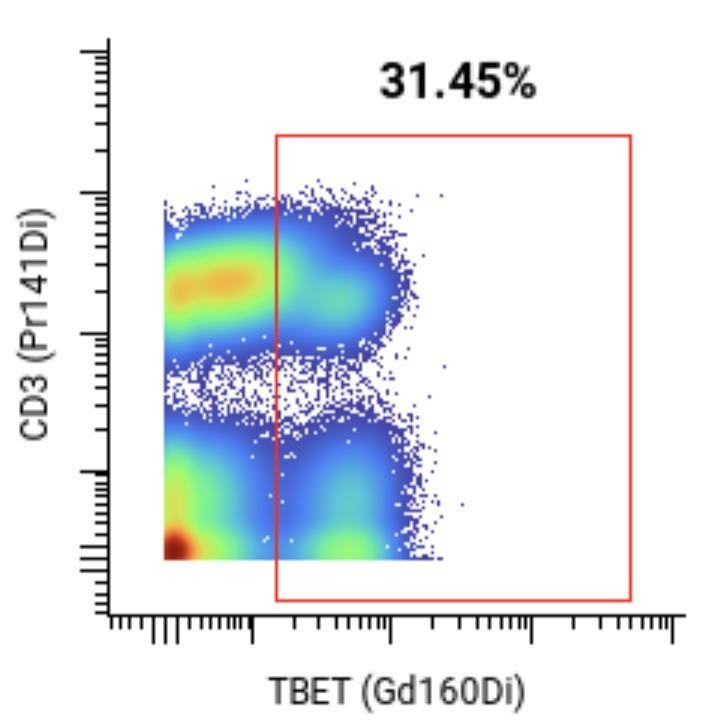
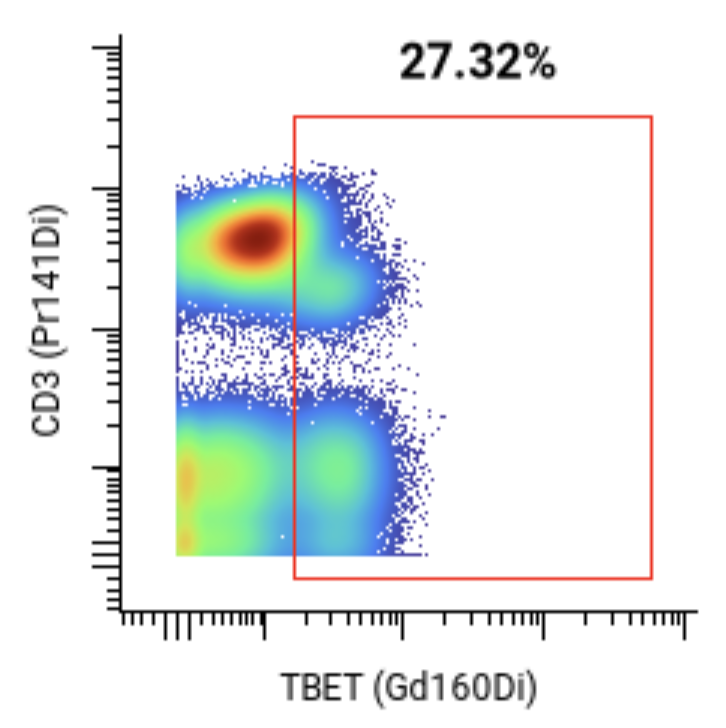
A 44-marker mass cytometry panel was applied to assess T, B, NK, myeloid, and other key immune cell populations
Instrument
Cytometry analysis was performed on a CyTOF® Helios.
.png)